Oxford Cancer Biomarkers achieves CE mark for OncoProg ® digital pathology computational software product

OXFORD, UK – July 19th, 2021 – Oxford Cancer Biomarkers (OCB), a pioneer in precision medicine, today announced it has received a CE mark for OncoProg ®, its cutting-edge digital pathology prognosis software that provides a more complete understanding of how aggressive a tumour is likely to be. This is the second CE mark achieved by OCB following the earlier accreditation of ToxNav ®, the most comprehensive DPYD test available.
The CE mark for OncoProg ® represents an important regulatory validation for our flagship digital pathology product and is an important milestone for the team as we start work on the future iterations of the product.
OncoProg ® was until recently, known as ColoProg ® given its initial intention to be used only within colorectal cancer. However, research has now shown the computational pathology product will have applications in other cancers, such as prostate and breast. OncoProg ® therefore reflects the wider cancer pool application.
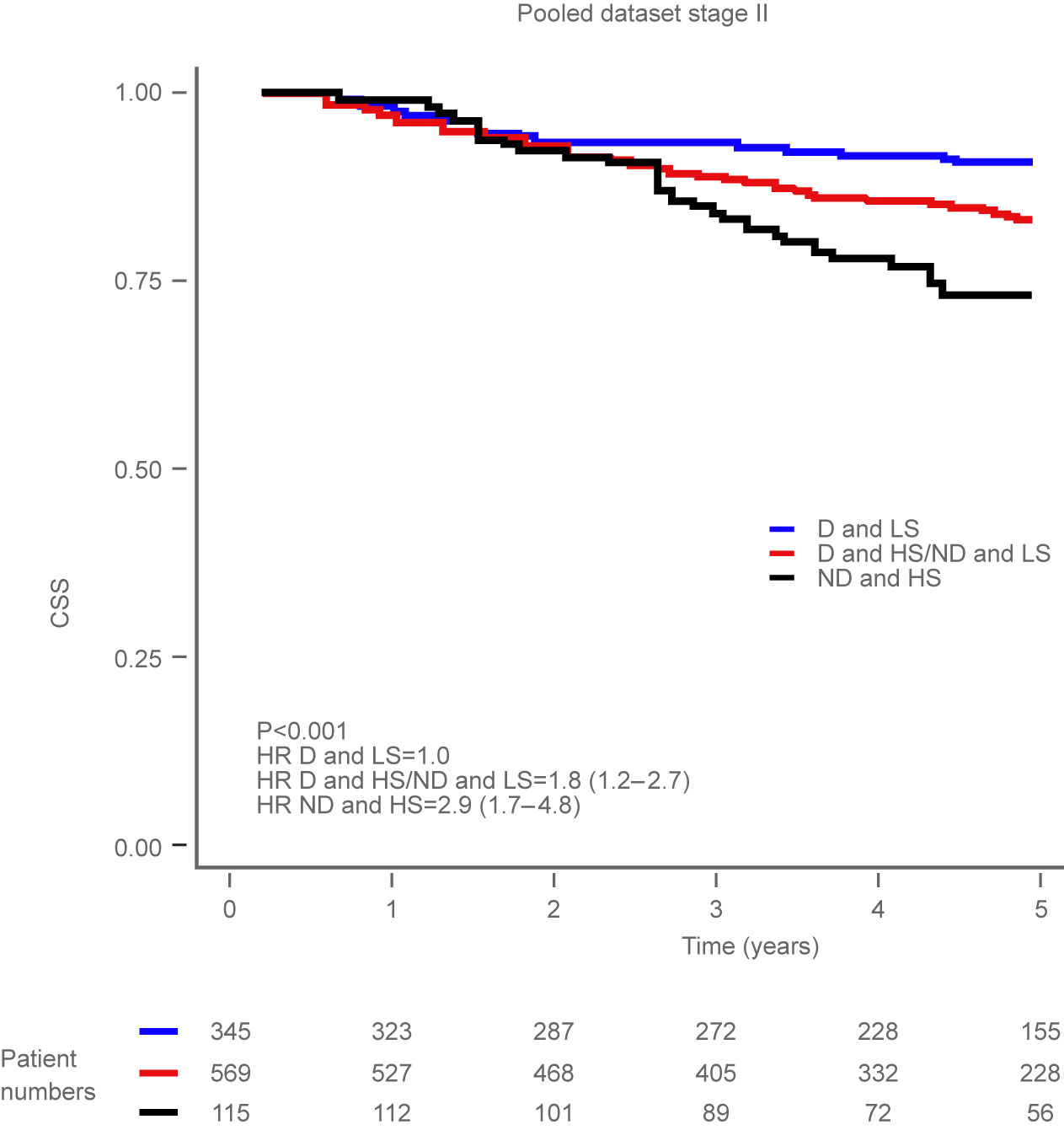
Our datasets from QAUSAR2 showing the distinct advantage of stratifying patients according to the presence or absence of the two biomarkers OncoProg is trained to detect. These two biomarkers are; tumour cell ploidy and stroma content (tumour microenvironment). You can see above that a patient who presents as non-diploid and high stroma content (black line) has worse cancer specific survival outcomes than a patient who presence as diploid and low stroma content (blue line). OncoProg combines these two crucial pieces of information in an easy to use software platform and generates a clinically actionable report for oncologists and pathologists to make better decision for a patients treatment pathway.
The OCB team are excited to continue the journey to enhance and improve care pathways in the field of oncology by developing OncoProg ® and other products like it to improve precision medicine capabilities and patient outcomes.
If you would like to know more about this technology, please get in touch with one of the team.
About OncoProg®
OncoProg ® determines the prognosis of Stage II colorectal cancer patients after treatment with fluoropyrimidine-based chemotherapy. The platform utilises digital pathology to examine specimens in order to determine DNA ploidy and tumour stroma content.
Biomarkers are combined to stratify patients into categories informing on risk of recurrence:
- High risk (non-diploid and high stroma).
- Intermediate risk (non-diploid and low stroma OR diploid and high stroma).
- Low risk (diploid and low stroma).
OncoProg ® provides clinicians and patients with information, that together with other relevant clinical parameters such as co-morbidities and performance status, can help assist in the choice of chemotherapy treatment regimens after initial surgery. OncoProg ® will eventually be compatible with other types of cancer and will be driven by artificial intelligence.
About Oxford Cancer Biomarkers
Oxford Cancer Biomarkers started as a spin off from the University of Oxford. The aim of the company was to discover and develop biomarkers using screening platforms to advance personalised medicine within oncology, specifically colorectal cancer.
Since then, the company has built a world class development team consisting of industry professionals, leading scientists and oncologists whilst maintaining strong links to the University of Oxford. Together, we have launched our first product – ToxNav ® – and are positioned to launch OncoProg ® into the UK this year (2021). Our vision is to be the leading pioneer in biomarker technology and deploy artificial intelligence that places individual patients at the heart of cancer care.
Contact
Oxford Cancer Biomarkers Ltd
Tel: +44 1865 784743