Professors David and Rachel Kerr’s proposed colorectal cancer treatment guidelines for patients during the COVID-19 era.
AIMS
- To reduce the comorbidity of chemotherapy and decrease the risk of our patients dying from COVID-19, weighing this up against their potential for benefit from chemotherapy
- To reduce the burden on the chemotherapy units at a time of great pressure
METHODS
We have modified the guidelines in such a way that we believe will decrease the TOTAL number of patients receiving chemotherapy (particularly in the adjuvant setting), and also to reduce the immune impact overall of chemotherapy on these patients, by changing doublet chemotherapy to single agent chemotherapy for some groups; and by changing to combinations involving capecitabine, rather than bolus and infusional 5FU for other patients; and finally by making reasonable dose reductions upfront to reduce the risk of cycle 1 complications. By changing from push and pump 5FU to capecitabine for the vast majority of patients, we will both reduce the rates of neutropenia, and will also decrease throughput through chemo out patient unit and reduce requirement for weekly line flushing, pump disconnections etc.
We will continue to use ToxNav as usual as a genetic screen for DYPD /ENOSF1 SNPs to identify patients at high risk of fluropyrimidine toxicity
RECOMMENDATIONS
ADJUVANT
| STAGE 2 | STAGE 3 | ||||
| Age / fitness | T3N0 | T4N0 | T3N1 | T4 N1/2 | T3 N2 |
| Under 70 and fit | Discuss pros and cons Cape alone 6/12 | Discuss pros and cons Cape alone 6/12 | Discuss pros and cons Cape alone 6/12 | Cape 0x 3/12 | Cape Ox 3/12 |
| Over 70 or under 70 and significant comorbid conditions | NO chemo | NO chemo or Cape* alone 6/12 | NO chemo or Cape* alone 6/12 | Cape* Ox* 3/12 | Cape* Ox* 3/12 |
| Over 70 and significant comorbid conditions | No chemo | No chemo | No chemo | NO chemo or Discuss Cape* Ox* 3/12 | NO chemo or Discuss Cape* Ox* 3/12 |
*If over 70 or other comorbid conditions that contribute to COVID-19 risk of morbidity and death, give reduction in cape of down to 80% standard dose and reduction in Ox down to 80% std dose. If either of these apply and also the patient suffers from mild to moderate renal impairment, then give 60% std dose cape
Any patients who are presently undergoing adjuvant chemotherapy should have a discussion about continuing with this, given the changes outlined above, especially patients who were originally pencilled in to receive 6 months of treatment, or patients in COVID-19 high risk groups
ADVANCED
Which regime?
Cape Ox should be the default backbone chemotherapy (rather than FOLFOX) in order to decrease the stress on infusion unit.
Cape Iri should be considered rather than FOLFIRI. However, in order to increase safety, we should dose reduce the capecitabine and the irinotecan, both to 80%, in all patient groups, and perhaps the capecitabine further to 60% in those over the age of 70 or with significant comorbid conditions.
Treatment breaks
Full treatment breaks should be considered after 3/12 of Rx in most patients with lower volume, more indolent disease. Treatment de-intensification to cape alone should be used in those with higher volume disease (eg more than 50% of liver replaced by tumour) at the beginning of treatment.
Deferring the start of any chemotherapy
Some older patients, or those with significant other comorbidity (ie who will be at increased risk of COVID-19 complications and death) who have low volume disease, such as a couple of small lung metastases, or a single liver metastasis, diagnosed more than 12 months since adjuvant chemotherapy, may decide to defer any chemotherapy for a period of time: In which case we would rescan at 3/12 and discuss treatment at that point. Some of these patients will be eligible for other interventions, such as resection / ablation / SBRT. However, it will be important to consider equally, the pressures upon these other services during this unprecedented time.
CHEMOTHERAPY AFTER RESECTION OF METASTASES
Given the lack of evidence, and the present extenuating circumstances, we would NOT recommend any chemotherapy in this setting.
ANYTHING ELSE WE CAN DO TO IMPROVE PROGNOSTICATION AND SELECTION OF PATIENTS
David Kerr and team have been developing novel prognostic markers that may well help us to select most appropriately which patients should have chemotherapy and which chemotherapy they should receive. One of these tests, developed from studying 1000s of patients across a number of large adjuvant studies, is ready to pilot.
Below is an excerpt from the background to this work; and shows some of the survival curves. We shall see if we can put together an Ethics application that will allow us to start piloting these prognostic markers in our group of patients which will give us further evidence on which to make our important adjuvant chemotherapeutic decisions.
BACKGROUND TO HISTIOTYPING
It has become clear that the traditional pathological biomarkers of T, N, V and L status do not adequately stratify the stage 2 and stage 3 colon cancer patients and therefore cannot give detailed enough information to allow clinicians to make coherent decisions about the type and duration of adjuvant chemotherapy, or even whether such chemotherapy should be administered at all. There are frequent unresolved discussions about whether individual patients with stage 2 disease should have any chemotherapy at all; and whether ‘lower risk’ stage 3 patients should be treated in a way more similar to stage 2 patients and not receive oxaliplatin. This has led to quite different patterns of clinical treatment between countries, between different cancer centres in the same country, and in some cases, divergence between different clinicians practising in the same cancer centre.
HISTIOTYPING is a newly developed test which utilized deep learning and digital analysis to develop a fully-automated prognostic system for primary colorectal cancer patients using scanned conventional haematoxylin and eosin stained sections. More than 12,000,000 image tiles were used to train a total of 10 convolutional neural networks, purpose-built for classifying supersized heterogeneous images. A total of 2473 patients were used for training and tuning, and the resulting ensemble system of the 10 networks was tested on 920 patients and finally validated in 1122 patients.
Histiotyping provided a hazard ratio for poor vs. good prognosis of 3·04 (95% confidence interval, 2·07-4·47; p<0·0001) in multivariable analysis of cancer-specific survival in the validation cohort, when adjusting for established prognostic markers significant in univariable analyses; pN stage, pT stage, lymphatic invasion and venous vascular invasion.
We combined stage and histiotype score to produce three risk groups, which are sufficiently well stratified to support potentially different therapeutic approaches
Low risk – stage II Histio low and intermediate + stage III N1 Histio low and intermediate (no T4 disease)
Intermediate risk – stage II Histio High + stage III N1 Histio High + N2 Histio low + T4 Histio low
High risk –Stage III N2 Histio Intermediate and High; T4 Histio Intermediate and High
4.2% (6 of 144) low risk patients suffered cancer-specific death. 5-year cancer-specific survival was 94.3% (95% CI, 87.3%-97.5%), and 5-year overall survival was 80.8% (95% CI, 71.5%-87.4%).
19.5% (23 of 118) intermediate risk patients suffered cancer-specific death. 5-year cancer-specific survival was 77.3% (95% CI, 66.8%-84.8%), and 5-year overall survival was 57.7% (95% CI, 46.0%-67.8%).
45.8% (121 of 264) high risk patients suffered cancer-specific death. 5-year cancer-specific survival was 46.3% (95% CI, 39.2%-53.1%), and 5-year overall survival was 40.5% (95% CI, 33.6%-47.2%).
Figure 1. KM Cancer specific survival plot with 95% confidence intervals (green – low risk; blue – intermediate risk; red – high risk)
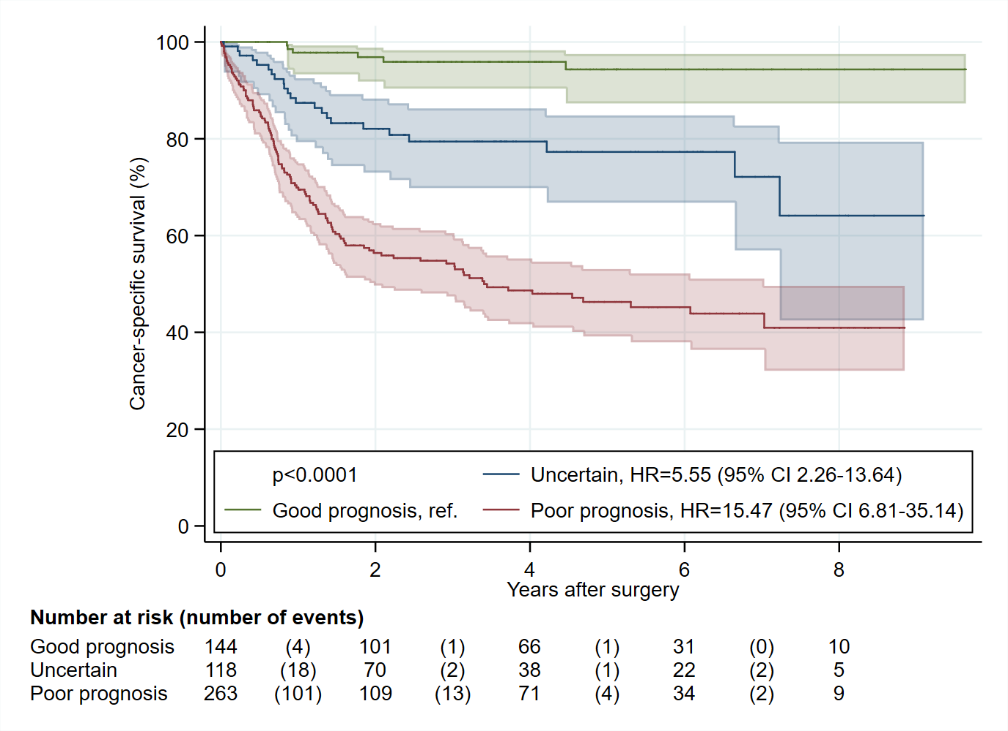
.
Such data should allow us to make better treatment decisions for our patients in the adjuvant setting as we adapt to practise during the COVID-19 pandemic.
Commentary of this proposed treatment guideline was been published on WebMD
Oxford Cancer Biomarkers are pleased to announce the rebranding of our ColoProg ® product to OncoProg ®
OXFORD, UK – April 21st, 2021 – OncoProg ® is a cutting-edge digital pathology prognosis tool that provides a more complete understanding of how aggressive a patient’s colorectal cancer is likely to be. OncoProg ® consists of proprietary software which combines a unique algorithm in which colorectal cancer specimens are assessed from patients who have undergone removal of their primary tumours. Tumour ploidy and stromal components are identified and, using a patented system, a risk of recurrence score calculated. This system links the prognostic algorithm to a method for measuring all the contributory elements in a single colorectal cancer formalin fixed paraffin embedded (FFPE) sample.
The change from ColoProg ® to OncoProg ® has arisen due to early data now demonstrating that the technology shows promise in more than one cancer type. Oxford Cancer Biomarkers is a pioneer in precision medicine techniques. Following the successful launch of our pharmacogenomic ToxNav ® DPYD test, the company is working hard to deploy the first iteration of OncoProg ® to drastically enhance the decision-making capabilities of Oncologists and improve patients’ lives.
If you would like to know more about this technology, please get in touch with one of the team.
About OncoProg ®
OncoProg ® determines the prognosis of Stage II colorectal cancer patients after treatment with fluoropyrimidine-based chemotherapy. The platform utilises digital pathology to examine specimens in order to determine DNA ploidy and tumour stroma content.
Biomarkers are combined to stratify patients into categories informing on risk of recurrence:
- High risk (non-diploid and high stroma)
- Intermediate risk (non-diploid and low stroma OR diploid and high stroma)
- Low risk (diploid and low stroma)
OncoProg ® provides clinicians and patients with information that, together with other relevant clinical parameters such as co-morbidities and performance status, can help assist in the choice of chemotherapy treatment regimens after initial surgery. OncoProg® is driven by artificial intelligence, will eventually be compatible for use with other types of cancer .
About Oxford Cancer Biomarkers
Oxford Cancer Biomarkers started as a spin off from the University of Oxford. The aim of the company was to discover and develop biomarkers using screening platforms to advance personalised medicine within oncology, specifically colorectal cancer.
Since then, the company has built a world class development team consisting of industry professionals, leading scientists and oncologists whilst maintaining strong links to the University of Oxford. Together, we have launched our first product – ToxNav ® – and are positioned to launch OncoProg ® into the UK this year (2021). Our vision is to be the leading pioneer in biomarker technology and deploy artificial intelligence that places individual patients at the heart of cancer care.
Contact:
Oxford Cancer Biomarkers Ltd
Tel: +44 1865 784743